



Hospital OEM/ODM guide for Adult Diapers With Tabs—how to go from prototype to production fast. Compliance, validation, private-label setup with LOVINHUG manufacturer.
This guide shows how to go from a working prototype to stable production with fewer surprises.

Going OEM? You bring the design, we build to print.
Going ODM? You leverage our platform, then customize fit, core build, and packaging.
Hospitals don’t care what you call it. They care about reliability, traceability, and post-market support. The legal “manufacturer” on the label owns the tech file, quality system alignment, vigilance, and recalls. If you private-label, you still carry the load.
Quick read:
| Topic | OEM (you own the spec) | ODM (you use our platform) | What hospitals expect |
|---|---|---|---|
| Design file & changes | You lead; factory executes change control | Joint; base spec locked; deltas documented | Clear revision history, no surprises mid-tender |
| Risk management | You drive; full FMEA trace | Shared; platform risk file + your deltas | Evidence of risk control across the product life |
| Quality agreement | Defines roles, audits, CAPA | Same, but references platform controls | Named contacts, response times, CAPA closure |
| Traceability | Lot trace to raw materials | Same | Pull the thread from ward back to supplier |
| Post-market | You collect feedback & trend | Shared | Complaints in, actions out, feedback loop closed |

A prototype is not a product. To cross the gap, lock the basics:
Design transfer means the line can run it repeatably. That’s BOM, specs, test methods, sampling plans, packaging bill, and work instructions synced.
| Step | What you do | Evidence you keep |
|---|---|---|
| Pilot run | Short runs, tweak tab tension, core distribution, emboss pattern | Run reports, rejects by failure type, rework notes |
| Tooling freeze | Lock cutting dies, emboss rollers, lamination pressure | Revision mark, redline, approval trail |
| Golden samples | Master pieces for QC & training | Signed master set, storage & pull policy |
| Release criteria | Clear pass/fail at incoming, in-process, final | Test methods, acceptance rules, AQL style sampling |
| Training | Operators + QC know the “why” not just the “how” | Training records, competency checks |
Big swings—like switching nonwoven lot or SAP grade—can shift performance. That’s why you need tiered supplier control:
Not every feature can be fully verified by checking the final diaper. Tab bond, side seam strength, or core stability across shifts—these need validated processes.
Set re-validation triggers: new mold, new adhesive, new substrate finish, or repeated drift in SPC charts.
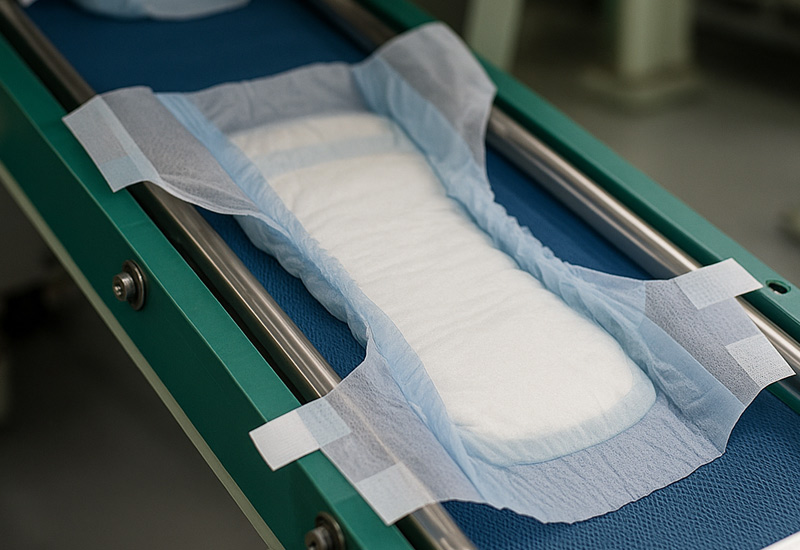
Hospitals order by case, handle by cart, stack in tight storerooms. Cartons need to survive transport, humidity, and quick grabs.
What to lock:
If you ship across continents (North America, Europe, MENA, SEA, LATAM, Oceania), align case marks to the biggest buyer’s requirement, then superset it for all.
You’ll hear these acronyms a lot:
Hospitals won’t ask for all of it, but large systems, regulators, and auditors might. So it will be better if you change logs are tidy.
Hospitals ask straight questions:
Make a one-pager: brand name, product family, certs, capacity ranges, and your contact window.
Different wards. Different pain points. Choose the right brief for the job.
LOVINHUG is a manufacturer focused on adult incontinence, with OEM/ODM for retailers, DTC brands, hospitals, and nursing homes. We run factory setups that are built for private-label briefs, pads, pull-ups, underpads, wipes, and ABDL lines. Reach covers North America, Europe, MENA, SEA, LATAM, and Oceania.
How we usually ramp with hospital buyers:
| Phase | Hospital buyer cares about | Your moves | Proof points |
|---|---|---|---|
| Concept | Fit, comfort, change time | Define user needs, cost envelope, packaging form | Brief, risk sketch |
| Prototype | Leak control, tab strength | Iterate core, ADL, guards, tabs | Test logs, nurse feedback |
| Pilot | Repeatability, QC clarity | Establish sampling, line settings, SPC | Pilot report, golden set |
| Validation | Stable outputs | IQ/OQ/PQ, re-validation triggers | Protocols, summaries |
| Launch | Supply, service | Artwork, case marks, service SLAs | Artwork approval, training sheet |
| Scale | Consistency across sites | Dual-sourcing, change control | Supplier scorecards, CAPA trail |
Certifications aren’t trophies; they’re passports. For adult briefs, keep CE alignment clean, maintain ISO 13485, use FSC where paper-based components are concerned, and stay current with new cGMP language. Put cert scans in your tender pack. Keep them updated before they expire.
Hospitals pay attention to three things:
Do this well and you win renewals. Keep doing it and you build a moat. LOVINHUG plays long-term: fewer promises, more deliveries.
Move from prototype to production with clear ownership. OEM gives spec control; ODM speeds ramp. Hospitals need reliability, traceability, and support. Lock design transfer, validate key processes, maintain DHF/DMR/DHR, and enforce change control.
If you wanna talk real specs, sample paths, or tender prep, fill the contact form—LOVINHUG will respond fast and straight. We’ll help you choose OEM vs ODM.